Tyler DeWitt | Atomic Structure: Discovery of the Neutron @tdewitt451 | Uploaded October 2012 | Updated October 2024, 1 day ago.
To see all my Chemistry videos, check out
socratic.org/chemistry
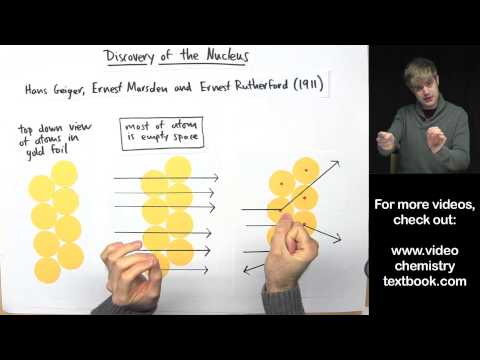
We know that there are protons and neutrons in the nucleus of an atom. How was the neutron discovered? James Chadwick found that atoms of Boron, Beryllium and Lithium released neutrons when they are bombarded with alpha particles. These neutrons cause protons to be ejected from paraffin wax. Neutrons are not gamma rays, because they have to have a lot of mass in order to knock protons out of paraffin with such speed.
To see all my Chemistry videos, check out
socratic.org/chemistry
We know that there are protons and neutrons in the nucleus of an atom. How was the neutron discovered? James Chadwick found that atoms of Boron, Beryllium and Lithium released neutrons when they are bombarded with alpha particles. These neutrons cause protons to be ejected from paraffin wax. Neutrons are not gamma rays, because they have to have a lot of mass in order to knock protons out of paraffin with such speed.