Tyler DeWitt | VSEPR Theory Part 2: Trigonal Bipyramidal Family @tdewitt451 | Uploaded July 2012 | Updated October 2024, 1 day ago.
To see all my Chemistry videos, check out
socratic.org/chemistry
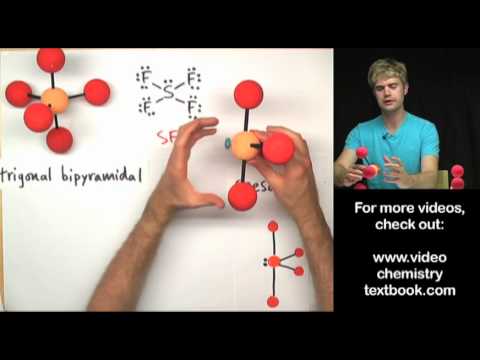
If the central atom in a molecular can make 5 bonds, the structure that it makes is based on the trigonal pyramidal shape. If the molecule has lone pairs or unshared electron pairs, the shape could be see saw, T-shaped, or linear. In this video, we'll look at diagrams of the VSEPR shapes, and examine bond angles for each structure.
To see all my Chemistry videos, check out
socratic.org/chemistry
If the central atom in a molecular can make 5 bonds, the structure that it makes is based on the trigonal pyramidal shape. If the molecule has lone pairs or unshared electron pairs, the shape could be see saw, T-shaped, or linear. In this video, we'll look at diagrams of the VSEPR shapes, and examine bond angles for each structure.