Tyler DeWitt | Discovery of the Nucleus: Rutherford's Gold Foil Experiment @tdewitt451 | Uploaded November 2012 | Updated October 2024, 1 day ago.
To see all my Chemistry videos, check out
socratic.org/chemistry
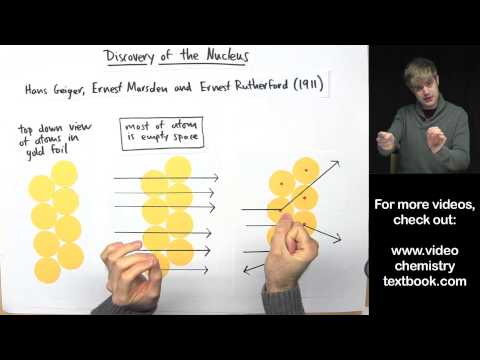
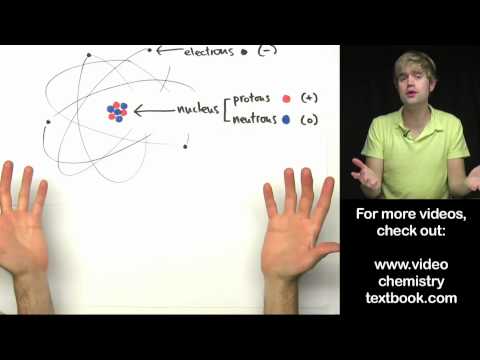
In 1911, Ernest Rutherford and his colleagues discovered the nucleus of the atom using their famous gold foil experiment. They shot alpha particles at a sheet of gold foil, and noticed that most went through, but some bounced back. This showed that atoms have a nucleus, and it disproved Thompson's plum pudding model of the atom.
To see all my Chemistry videos, check out
socratic.org/chemistry
In 1911, Ernest Rutherford and his colleagues discovered the nucleus of the atom using their famous gold foil experiment. They shot alpha particles at a sheet of gold foil, and noticed that most went through, but some bounced back. This showed that atoms have a nucleus, and it disproved Thompson's plum pudding model of the atom.