Tyler DeWitt | What's the Difference between Mass Number and Atomic Weight? @tdewitt451 | Uploaded October 2012 | Updated October 2024, 1 day ago.
Need help? Ask me your questions here:
vespr.org/videos/5130b7d29d53443c3bd593d8
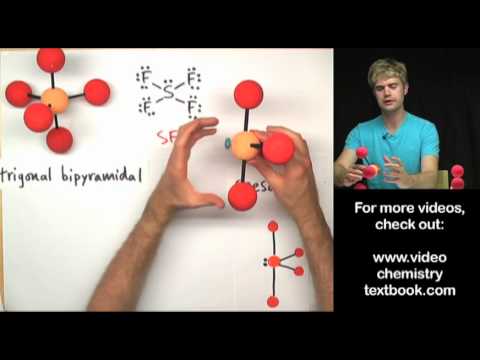
What's the difference between mass number and atomic weight? Mass number is the number of protons and neutrons in an atom, and it tells us about the mass of the atom in amu, or atomic mass units. Atomic weight is the average mass of all the isotopes of a certain type. It is a weighted average that takes into account the abundances of all of the different isotopes.
Need help? Ask me your questions here:
vespr.org/videos/5130b7d29d53443c3bd593d8
What's the difference between mass number and atomic weight? Mass number is the number of protons and neutrons in an atom, and it tells us about the mass of the atom in amu, or atomic mass units. Atomic weight is the average mass of all the isotopes of a certain type. It is a weighted average that takes into account the abundances of all of the different isotopes.