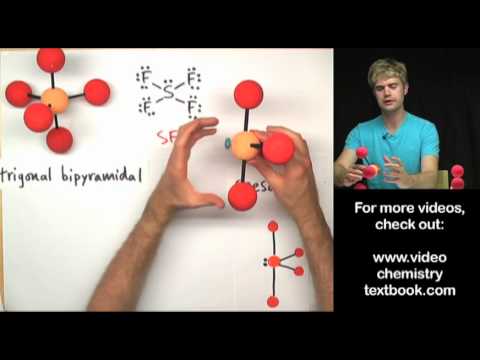
Tyler DeWitt | Temperature and Solubility: Solids and Gases @tdewitt451 | Uploaded May 2021 | Updated October 2024, 1 day ago.
We'll look at the relationship between temperature and solubility for solids and gases. How much of a solid or gas solute can dissolve in a certain amount of liquid solvent? We will see the pattern or trend. For solids dissolving in water, it is a generally direct relationship. Although there are some exceptions, as temperature increases, solubility increases as well. For gas dissolving in liquid, it is an inverse relationship: as temperature increases, solubility decreases. We will look at graphs and charts, and also see real world examples. The amount of sugar that can dissolve in coffee (or water) increases as temperature increases. However, soda gets more bubbly as temperature increases, because it is less soluble. Also, less oxygen can dissolve in lakes and bodies of water at higher temperature. This can be caused by thermal pollution, when factories or power plants dump hot water into the environment.
We'll look at the relationship between temperature and solubility for solids and gases. How much of a solid or gas solute can dissolve in a certain amount of liquid solvent? We will see the pattern or trend. For solids dissolving in water, it is a generally direct relationship. Although there are some exceptions, as temperature increases, solubility increases as well. For gas dissolving in liquid, it is an inverse relationship: as temperature increases, solubility decreases. We will look at graphs and charts, and also see real world examples. The amount of sugar that can dissolve in coffee (or water) increases as temperature increases. However, soda gets more bubbly as temperature increases, because it is less soluble. Also, less oxygen can dissolve in lakes and bodies of water at higher temperature. This can be caused by thermal pollution, when factories or power plants dump hot water into the environment.