Tyler DeWitt | What are the Diatomic Elements? @tdewitt451 | Uploaded February 2012 | Updated October 2024, 18 hours ago.
To see all my Chemistry videos, check out
socratic.org/chemistry
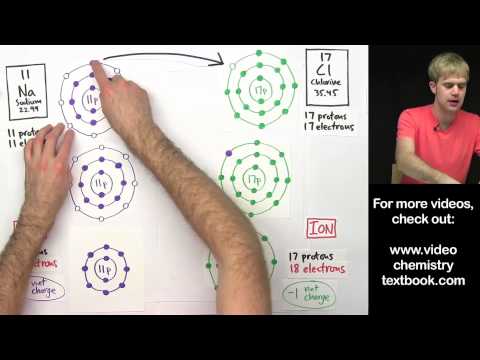
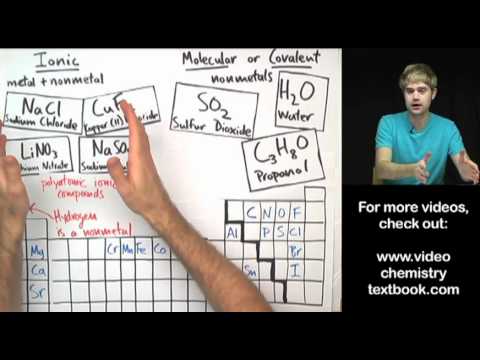
Learn what the diatomic elements are, how to remember them, and what they look like. Diatomic elements are all gases, and they form molecules because they don't have full valence shells on their own. The diatomic elements are: Bromine, Iodine, Nitrogen, Chlorine, Hydrogen, Oxygen, and Fluorine. Ways to remember them are: BrINClHOF and Have No Fear Of Ice Cold Beer.
To see all my Chemistry videos, check out
socratic.org/chemistry
Learn what the diatomic elements are, how to remember them, and what they look like. Diatomic elements are all gases, and they form molecules because they don't have full valence shells on their own. The diatomic elements are: Bromine, Iodine, Nitrogen, Chlorine, Hydrogen, Oxygen, and Fluorine. Ways to remember them are: BrINClHOF and Have No Fear Of Ice Cold Beer.