Tyler DeWitt | AP® Chemistry: Bonding, Hybridization, Intermolecular Forces, Enthalpy @tdewitt451 | Uploaded April 2022 | Updated October 2024, 20 hours ago.
tdwscience.com/apchem
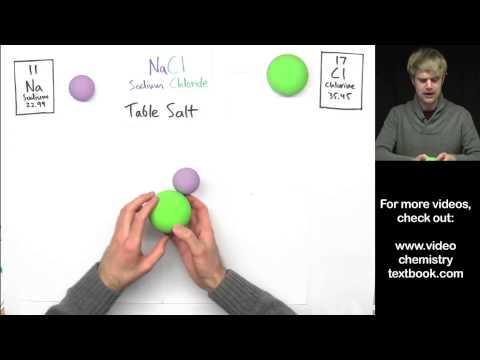
This video covers is an example for a long format free response question for the AP® Chemistry exam. It covers a variety of topics that include: Lewis Structures, Hybridization, Bond Angles, Intermolecular Forces, Hydrogen Bonding, Dipole-Dipole Interactions or Attractions, Boiling Point, Melting Point, Enthalpy of Formation Calculations, and Stoichiometry. The Lewis Structures include alkanes, alcohols, carboxylic acids, and aldehydes. We discuss hybridization for molecules with sp, sp2, and sp3 states. Bond angles are for VSEPR geometries of linear (180º), trigonal planar (120º), and tetrahedral (109.5º). For the stoichometry calculations, we calculate the enthalpy of combustion and enthalpy of reaction, by summing the standard enthalpies of the products and reactants, using a table of enthalpy values.
tdwscience.com/apchem
This video covers is an example for a long format free response question for the AP® Chemistry exam. It covers a variety of topics that include: Lewis Structures, Hybridization, Bond Angles, Intermolecular Forces, Hydrogen Bonding, Dipole-Dipole Interactions or Attractions, Boiling Point, Melting Point, Enthalpy of Formation Calculations, and Stoichiometry. The Lewis Structures include alkanes, alcohols, carboxylic acids, and aldehydes. We discuss hybridization for molecules with sp, sp2, and sp3 states. Bond angles are for VSEPR geometries of linear (180º), trigonal planar (120º), and tetrahedral (109.5º). For the stoichometry calculations, we calculate the enthalpy of combustion and enthalpy of reaction, by summing the standard enthalpies of the products and reactants, using a table of enthalpy values.