Tyler DeWitt | Hydrogen Bonding and Common Mistakes @tdewitt451 | Uploaded June 2012 | Updated October 2024, 19 hours ago.
To see all my Chemistry videos, check out
socratic.org/chemistry
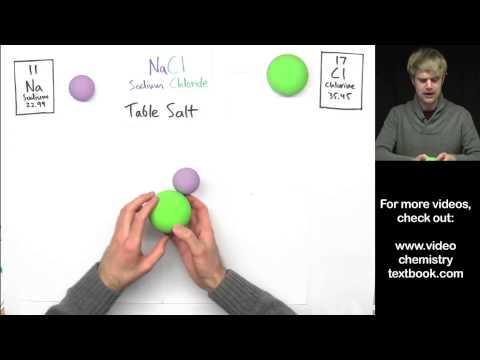
Hydrogen bonding can be so confusing, and in this video we talk about some common mistakes. Hydrogen bonds are intermolecular forces between molecules. They form because one atom has a high electronegativity, so it gets a partial negative charge, and the hydrogen gets a partial positive charge.
To see all my Chemistry videos, check out
socratic.org/chemistry
Hydrogen bonding can be so confusing, and in this video we talk about some common mistakes. Hydrogen bonds are intermolecular forces between molecules. They form because one atom has a high electronegativity, so it gets a partial negative charge, and the hydrogen gets a partial positive charge.