Tyler DeWitt | Ionic vs. Molecular @tdewitt451 | Uploaded May 2012 | Updated October 2024, 19 hours ago.
To see all my Chemistry videos, check out
socratic.org/chemistry
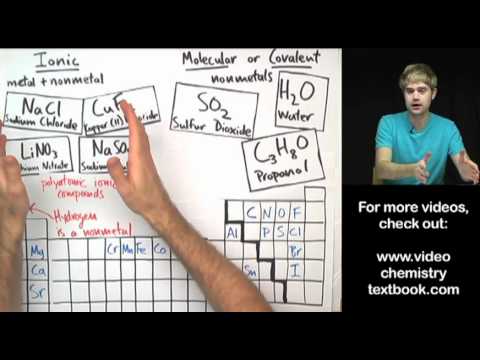
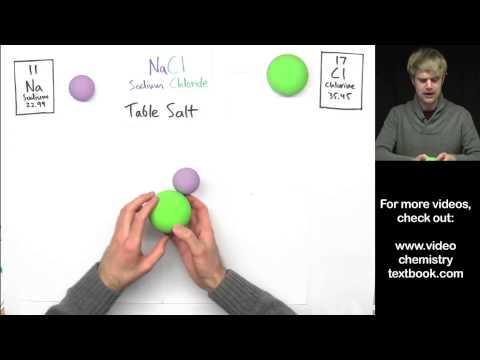
How can you tell the difference between compounds that are ionic and molecular (also known as covalent)? It has to do with the elements that make them up: ionic compounds are made of metals and nonmetals, and molecular (or covalent) compounds are made of nonmetals. We'll learn how they bond differently: in covalent compounds, the atoms share electrons, and in ion compounds, atoms steal electrons and then opposite charges attract. Ionic and molecular (covalent) compounds also look different at the microscopic level: covalent and molecular compounds exist in molecules, while ionic compounds are organized in lattice structures.
To see all my Chemistry videos, check out
socratic.org/chemistry
How can you tell the difference between compounds that are ionic and molecular (also known as covalent)? It has to do with the elements that make them up: ionic compounds are made of metals and nonmetals, and molecular (or covalent) compounds are made of nonmetals. We'll learn how they bond differently: in covalent compounds, the atoms share electrons, and in ion compounds, atoms steal electrons and then opposite charges attract. Ionic and molecular (covalent) compounds also look different at the microscopic level: covalent and molecular compounds exist in molecules, while ionic compounds are organized in lattice structures.