Tyler DeWitt | Molarity Practice Problems (Part 2) @tdewitt451 | Uploaded September 2012 | Updated October 2024, 1 day ago.
To see all my Chemistry videos, check out
socratic.org/chemistry
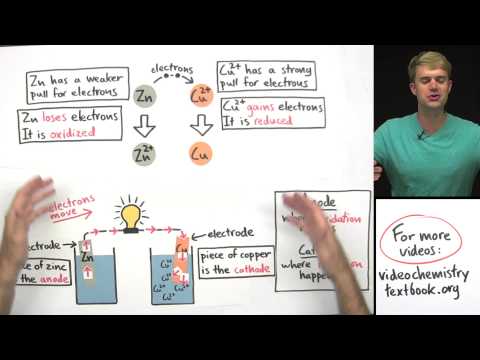
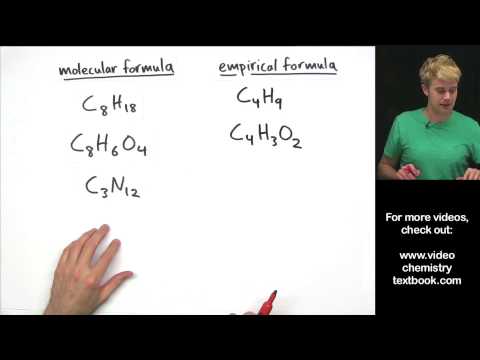
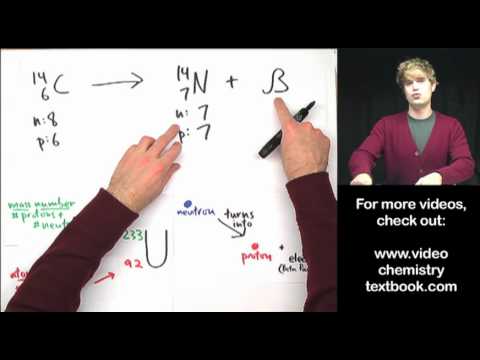
Use molarity to convert between mass and volume in a solution. In this video, we'll look at how to use molarity as a conversion factor. If you know the molarity, you can solve for either the number of moles or the volume of a solution. Also, molarity is a ratio that describes the moles of solute per liter of solution.
To see all my Chemistry videos, check out
socratic.org/chemistry
Use molarity to convert between mass and volume in a solution. In this video, we'll look at how to use molarity as a conversion factor. If you know the molarity, you can solve for either the number of moles or the volume of a solution. Also, molarity is a ratio that describes the moles of solute per liter of solution.