Tyler DeWitt | Electron Capture @tdewitt451 | Uploaded January 2012 | Updated October 2024, 1 day ago.
To see all my Chemistry videos, check out
socratic.org/chemistry
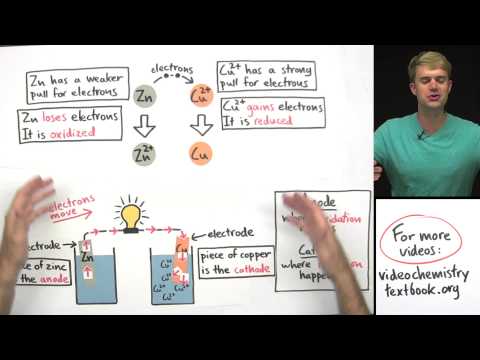
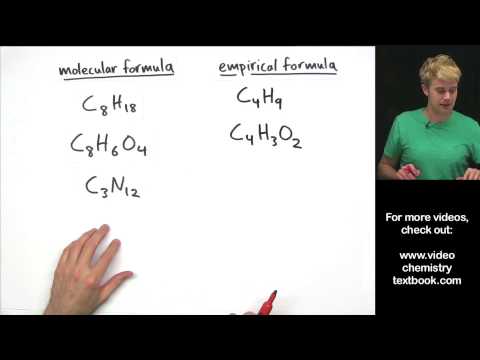
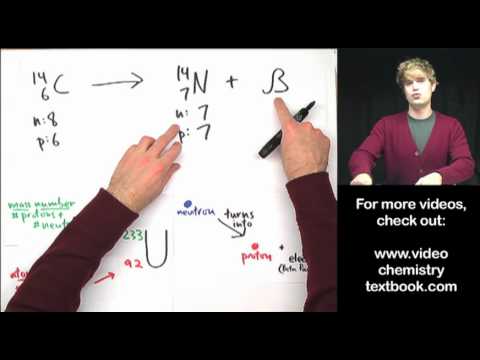
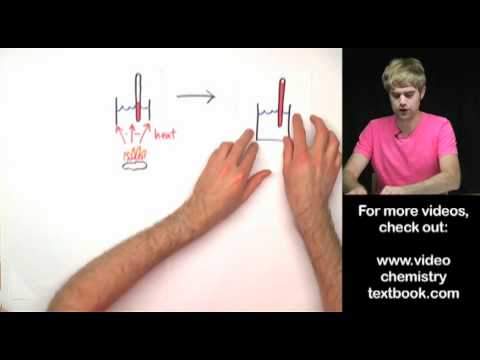
We introduce electron capture and do some practice example problems. Electron capture is a nuclear decay process. It turns a proton into a neutron, lowering the atomic number, but keeping the mass number the same. It creates gamma rays in the process. Electron capture is similar to alpha decay, beta decay, and positron decay.
To see all my Chemistry videos, check out
socratic.org/chemistry
We introduce electron capture and do some practice example problems. Electron capture is a nuclear decay process. It turns a proton into a neutron, lowering the atomic number, but keeping the mass number the same. It creates gamma rays in the process. Electron capture is similar to alpha decay, beta decay, and positron decay.