Quick Biochemistry Basics | Henderson Hasselbalch equation. @quickbiochemistrybasics | Uploaded 6 years ago | Updated 1 day ago
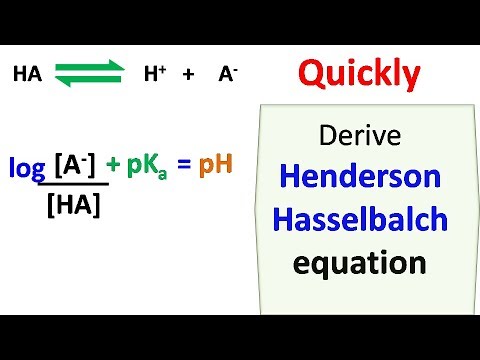
Henderson Hasselbalch equation describes the derivation of pH as a measure of acidity (using pKa, the negative log of the acid dissociation constant) in biological and chemical systems. HendersonHasselbalch equation is also useful for estimating the pH of a buffer solution and finding the equilibrium pH in acid-base reactions (it is widely used to calculate the isoelectric point of proteins).
Henderson Hasselbalch equation describes the derivation of pH as a measure of acidity (using pKa, the negative log of the acid dissociation constant) in biological and chemical systems. HendersonHasselbalch equation is also useful for estimating the pH of a buffer solution and finding the equilibrium pH in acid-base reactions (it is widely used to calculate the isoelectric point of proteins).