Tyler DeWitt | Thermochemical Equations Practice Problems @tdewitt451 | Uploaded April 2012 | Updated October 2024, 4 hours ago.
Need help? Ask me your questions here:
vespr.org/videos/5130b7d19d53443c3bd5938b
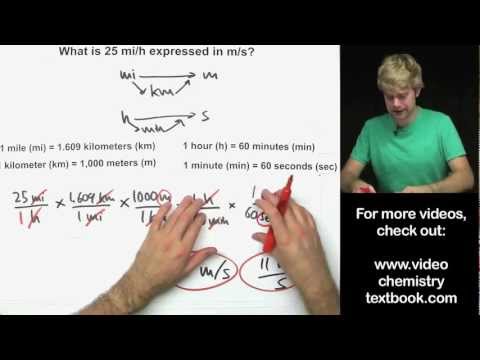
How much heat gets released or absorbed in a chemical reaction? We'll learn how to calculate this. We will use molar mass and conversion factors to figure out the enthalpy change in exothermic and endothermic reactions, which are represented by thermochemical equations.
Need help? Ask me your questions here:
vespr.org/videos/5130b7d19d53443c3bd5938b
How much heat gets released or absorbed in a chemical reaction? We'll learn how to calculate this. We will use molar mass and conversion factors to figure out the enthalpy change in exothermic and endothermic reactions, which are represented by thermochemical equations.