Tyler DeWitt | Introduction to Balancing Chemical Equations @tdewitt451 | Uploaded September 2015 | Updated October 2024, 1 day ago.
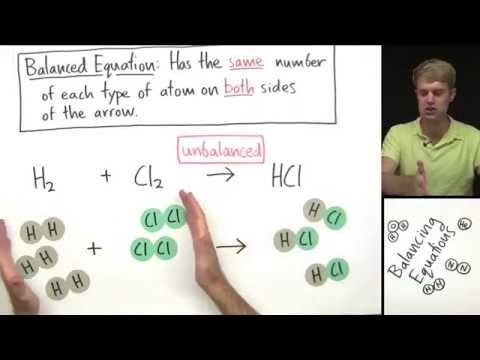
How to balance chemical equations. We'll start out with examples that show the concepts behind balancing chemical equations. We will start with a word equation, and then write a chemical equations, and then visualize the atoms and molecules and how they change. To figure out if the equations is balanced, we look at the number and type of atoms on each side of the arrow. If the number and type of atom is not the same on both sides, the equation in unbalanced. We need to change the number of one or more of the compounds in order to get the atoms to balance. We do this by placing coefficients (numbers) in front of each of the compounds. When balancing equations, you cannot ever change the subscripts of a compound.
How to balance chemical equations. We'll start out with examples that show the concepts behind balancing chemical equations. We will start with a word equation, and then write a chemical equations, and then visualize the atoms and molecules and how they change. To figure out if the equations is balanced, we look at the number and type of atoms on each side of the arrow. If the number and type of atom is not the same on both sides, the equation in unbalanced. We need to change the number of one or more of the compounds in order to get the atoms to balance. We do this by placing coefficients (numbers) in front of each of the compounds. When balancing equations, you cannot ever change the subscripts of a compound.